All published articles of this journal are available on ScienceDirect.
Cleanse, Control and Calm: 3C for Simpler Self-care for Atopic Dermatitis
Abstract
Atopic dermatitis (AD) is a common, chronic inflammatory skin condition affecting a substantial number of individuals globally. Managing AD is a common challenge not only for patients but also for primary care practitioners (PCPs), as they are frequently called to help/care for patients who are seeking relief from its distressing symptoms. This paper presents a concise guide to an eczema self-care routine, which may serve as a valuable resource for PCPs seeking to improve long-term AD management in their patients. We discuss the importance of addressing skin barrier dysfunction and highlight using emollients with active ingredients as fundamental components of AD care. Additionally, we introduce an easy-to-follow 3C (Cleanse, Control, Calm) approach to daily AD self-care that strengthens skin barrier function, supports steroid-sparing, and helps break the cycle of symptomatic treatment. By equipping PCPs with up-to-date knowledge of AD pathophysiology and evidence-based management strategies, this guide seeks to enhance the quality of care provided by PCPs, thereby improving the well-being of individuals living with AD.
1. INTRODUCTION
Atopic dermatitis (AD) is a highly prevalent chronic inflammatory skin condition, affecting approximately 2.6% of the global population, including 2.0% of adults and 4.0% of children worldwide [1]. AD is characterised by dryness (xerosis), redness (erythema), and intense itching (pruritus), which promotes a vicious “itch-scratch cycle” that exacerbates inflammation and contributes to disease progression [2-4]. The burden of AD extends beyond physical symptoms, affecting individuals’ quality of life (QoL) by disrupting sleep, causing social difficulties, and limiting daily activities [3, 5].
(Fig. 1) Provides an overview of the major challenges AD patients face, including difficulties in diagnosis, treatment efficacy/safety and adherence challenges, and gaps in long-term disease management. Diagnosis remains a significant unmet need, especially for atypical cases, as it depends heavily on healthcare professionals’ clinical expertise [6, 7]. A 2022 market research study in Thailand found that 47% of adults with AD were unaware of their condition, highlighting the risk of delayed or inaccurate diagnosis, which in turn can contribute to suboptimal treatment strategies, worsened symptoms, and reduced QoL.
Following diagnosis, AD management typically follows a stepped-care approach. First-line management consists of emollients, followed by topical corticosteroids (TCS), topical calcineurin inhibitors (TCIs), or topical phosphodiesterase 4 inhibitors for persistent symptoms [8-10]. In moderate to severe cases, systemic therapies such as systemic immunosuppressants, biologics, Janus kinase (JAK) inhibitors, and ultraviolet B (UVB) phototherapy may be necessary [8-10]. While these treatments provide symptomatic relief, concerns remain about long-term reliance on certain options. For instance, prolonged or inappropriate TCS use has been associated with adverse effects, such as skin thinning, enlarged blood vessels, and potential systemic absorption; therefore, potential long-term effects should be considered [11-14]. In addition to safety concerns, experiences with the adverse effects of pharmacological treatments may reduce treatment adherence [15], further contributing to suboptimal long-term management. Such management approaches may also impact long-term treatment affordability, while access to specialised treatment modalities such as UVB phototherapy may be limited (Fig. 1) [5].
Since repeated short-term symptomatic treatment does not fully address the chronic nature of AD, long-term management requires a comprehensive approach that extends beyond reactive treatment. Alongside “as-needed” symptomatic treatments, daily self-care plays a central role in maintaining remission and preventing flare-ups [16-18]. However, patients often report that they lack the resources, knowledge, skills, and/or confidence needed to effectively manage their condition on a daily basis (Fig. 1) [16]. While dermatology guidelines recommend evidence-based self-care practices, such as regular emollient use even during remission, these recommendations are often fragmented across multiple sources and not presented in a patient-friendly manner, making it challenging for patients to integrate them into a regimen that they can easily follow [8, 19-24].
Addressing this gap requires helping patients establish self-care routines, in addition to educational interventions to improve patients’ or caregivers’ understanding of the condition. Frontline primary care practitioners (PCPs), such as general practitioners and community pharmacists, are often consulted for day-to-day management of mild to moderate AD symptoms and are therefore well-positioned to reinforce effective self-care strategies [25-28]. Alongside their traditional roles in prescribing, dispensing, and advising on medication use, PCPs could also help promote certain aspects of self-care to AD patients.
In this paper, we aim to provide PCPs with relevant and practical information to support them in patient consultations for mild (SCORAD<25) to moderate (SCORAD<50) AD [29]. We explored the literature to retrieve articles on the following topics: (1) AD pathophysiology, treatment strategies, and self-care practices; (2) major dermatology guidelines and position statements on AD management; and (3) commonly used active ingredients in emollients (e.g., humectants, soothing/antipruritic agents). Methodological considerations for this narrative review included a content-led search strategy conducted in PubMed in 2023, supplemented by manual screening of reference lists from relevant articles. No restrictions were placed on publication date or study type. Based on the reviewed literature, we (i) address the importance of skin barrier dysfunction in AD, (ii) highlight the crucial role of emollients with active ingredients in AD management, and (iii) present the 3Cs (Cleanse, Control, Calm) approach for self-care. The 3Cs represent a simple, evidence-based daily self-care regimen that complements AD treatment while aligning with best-practice recommendations to help reduce treatment “cycling” and over-reliance on TCS use.
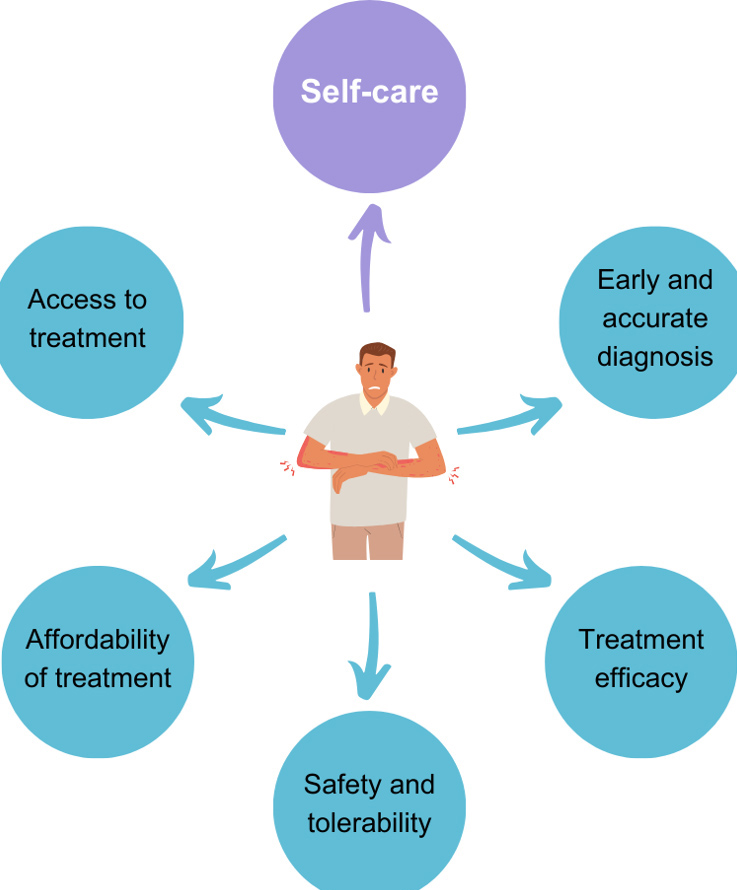
Unmet needs in mild/moderate atopic dermatitis (AD) management. An illustration of the major challenges in managing AD., emphasising the important role of consistent self-care practices to improve management outcomes alongside concerns of early and accurate diagnosis, treatment safety, tolerability, accessibility, affordability, and efficacy.
2. IMPORTANCE OF HEALTHY SKIN BARRIER FUNCTION IN AD MANAGEMENT
In a healthy state, the skin is a protective barrier against external factors such as mechanical, chemical, and microbial irritants [30]. The disruption of the skin barrier, primarily from defects in barrier integrity [31-34], leads to increased transepidermal water loss (TEWL), which indicates a rise in skin permeability and reduced protection against external irritants and allergens. Consequently, the entry of external irritants and allergens can trigger an immune response, promote inflammation, and ultimately result in AD flare-ups and skin lesions, all of which further compromise the skin barrier [30, 35]. If left untreated, this can lead to a relentless and vicious cycle, emphasising the importance of maintaining healthy skin barrier function to manage AD [36].
2.1. Factors Contributing to Skin Barrier Dysfunction in AD
Disruptions to skin barrier homeostasis can be due to a combination of factors. The outermost layer of the epidermis, known as the stratum corneum (SC), plays a pivotal role in maintaining skin integrity. It comprises multiple layers of flattened, non-nucleated corneocytes surrounded by numerous flat lamellar sheets containing ceramides, cholesterol, and free fatty acids (FFAs) [37, 38]. Alterations in the lipid content, composition, and organisation of the SC are leading causes of compromised skin barrier function observed in individuals with AD [39-41]. Exposure to external factors, such as irritants, allergens, microbial agents or extreme weather conditions, can also contribute to skin barrier dysfunction and exacerbate AD symptoms such as dryness and inflammation [3]. Moreover, in individuals with AD, the skin’s pH balance is often beyond the normal slightly acidic range of 4 to 6 [42]. This shift weakens the lipid barrier, increases protease activity, and impairs normal moisture retention [43]. Collectively, these factors lead to increased susceptibility to environmental irritants and vulnerability to further damage.
A healthy skin microbiome is equally crucial for maintaining immune homeostasis and preventing colonisation by pathogens [44, 45]. Studies consistently show that during AD flare-ups, the opportunistic pathogen Staphylococcus aureus significantly increases in relative abundance while diversity in the skin microbiota decreases, leading to dysbiosis [46-48]. This imbalance can lead to inflammatory cascades because of the complex interplay between skin commensals and immune effectors [4, 30, 49]. Studies in mouse models and clinical settings have demonstrated that antibiotic-based interventions targeting the skin microbiota can effectively reduce S. aureus levels, increase microbial diversity, and alleviate AD symptoms [50, 51], further emphasising the vital role of a healthy microbiome in AD management.
Recent research suggests that bacterial biofilms play a central role in various dermatological conditions; their relevance to skin barrier disruption in AD has also been recently proposed [reviewed in reference [52]. Biofilms within AD skin lesions can infiltrate sweat ducts, intensifying itching and causing additional skin barrier damage due to scratching, or can trigger the host’s immune system to activate self-destructive responses in its tissues [53, 54].
Given the interplay between pH balance, microbial dysbiosis, and biofilm formation, maintaining the skin’s natural acidic pH is vital. The elevated pH in the inflamed skin of AD patients favours pathogen colonisation and reduction in microbiome diversity [49, 55].
Taken together, addressing factors that cause skin barrier dysfunction and microbial dysbiosis is essential for effective long-term AD management.
3. ADDRESSING SKIN BARRIER DYSFUNCTION AND MICROBIAL DYSBIOSIS THROUGH THE USE OF EMOLLIENTS WITH ACTIVE INGREDIENTS
There has been an increasing interest in using non-medicated emollient preparations with active ingredients to target different mechanisms in AD pathophysiology [20]. These active ingredients can be classified according to their functions, including agents that strengthen the skin barrier, humectants, occlusive agents, soothing/antipruritic agents, and agents that support healthy microflora (Table 1).
3.1. Increasing Skin Hydration and Reducing TEWL with Anti-inflammatory Effects
Increasing the skin’s moisture content is vital and forms the foundation for AD management regimens. Emollients are topical preparations that help maintain skin hydration by combining humectants, which attract and retain water within the SC, and occlusives, which form a protective film to reduce TEWL [20]. Although basic emollients containing efficient humectants, such as glycerin and urea, can promote skin hydration and reduce TEWL, they lack active ingredients that can help address the underlying pathophysiological processes in AD [56-59]. Therefore, emollients containing soothing/antipruritic agents can further help alleviate the inflammation, itching, and discomfort associated with AD. Several soothing agents found to be effective in relieving itch include niacinamide , palmitoyl ethanolamide, stearyl glycyrrhetinate, glycyrrhetinic acid, and licochalcone A, and these are commonly found in emollients with active ingredients [60-68]. Current evidence supports the clinical efficacy of emollients with active ingredients in maintaining a healthy skin barrier and synergy with topical therapies during flare-ups [69]. For example, recent studies suggest that topical emollients with physiological lipids can replenish deficient skin lipids in individuals with AD, helping restore barrier integrity and improve skin health by promoting lamellar membrane production [70]. Some of these ingredients include ceramides, fatty acids, hyaluronic acid, dexpanthenol, squalane, and isostearyl isostearate (Fig. 2) [71-77].
| Category | Key Benefits | Ingredient Examples |
|---|---|---|
| Humectants | Promote hydration of the SC by attracting and retaining water. | Natural moisturising factors like amino acids, urea, lactic acids, and pyrrolidone carboxylic acid (PCA). Other moisturising factors like glycerin, hyaluronic acid, glycols (e.g., propylene glycol, polyethylene glycol [PEG], butylene glycol), sugars and sugar alcohols (e.g., fructose, glucose, inositol, mannitol, sorbitol). |
| Occlusive agents | Create a protective, hydrophobic layer on the skin surface, thus reducing TEWL and shielding the skin from external irritants. | Petrolatum, paraffin, beeswax, carnauba wax, lanolin, dimethicone, methicone, polysiloxane, cyclomethicone. |
| Skin barrier supporting agents | Restore and strengthen the skin barrier to enhance resilience and reduce water loss. | Skin identical lipids like ceramides, fatty acids (e.g., linoleic acid, stearic acid), sterols, cholesterol derivatives, squalane, triglycerides, isostearyl isostearate (ISIS). Essential fatty acid-rich oils like evening primrose oil, grape seed oil, safflower oil, canola oil, sunflower oil, flaxseed oil, almond oil, borage oil. |
| Soothing/antipruritic agents | Alleviate skin irritation, reduce redness and itchiness, and promote comfort by calming the skin and supporting its natural repair processes. | Vitamins A, E, B (e.g., niacinamide), dexpanthenol (provitamin B5), glycyrrhetinic acid, stearyl glycyrrhetinate, licochalcone A, aloe vera, oat extract, bisabolol, palmitoyl ethanolamide, polidocanol, menthoxypropanediol, cooling agents (e.g., menthol, camphor), tannins. |
| Healthy microflora supporting agents | Promote the restoration and balance of a healthy skin microbiome while inhibiting skin colonisation by pathogenic microorganisms. | α-glucan oligosaccharide, alditols (e.g., xylitol), lysates (e.g., Vitreoscilla filiformis). |
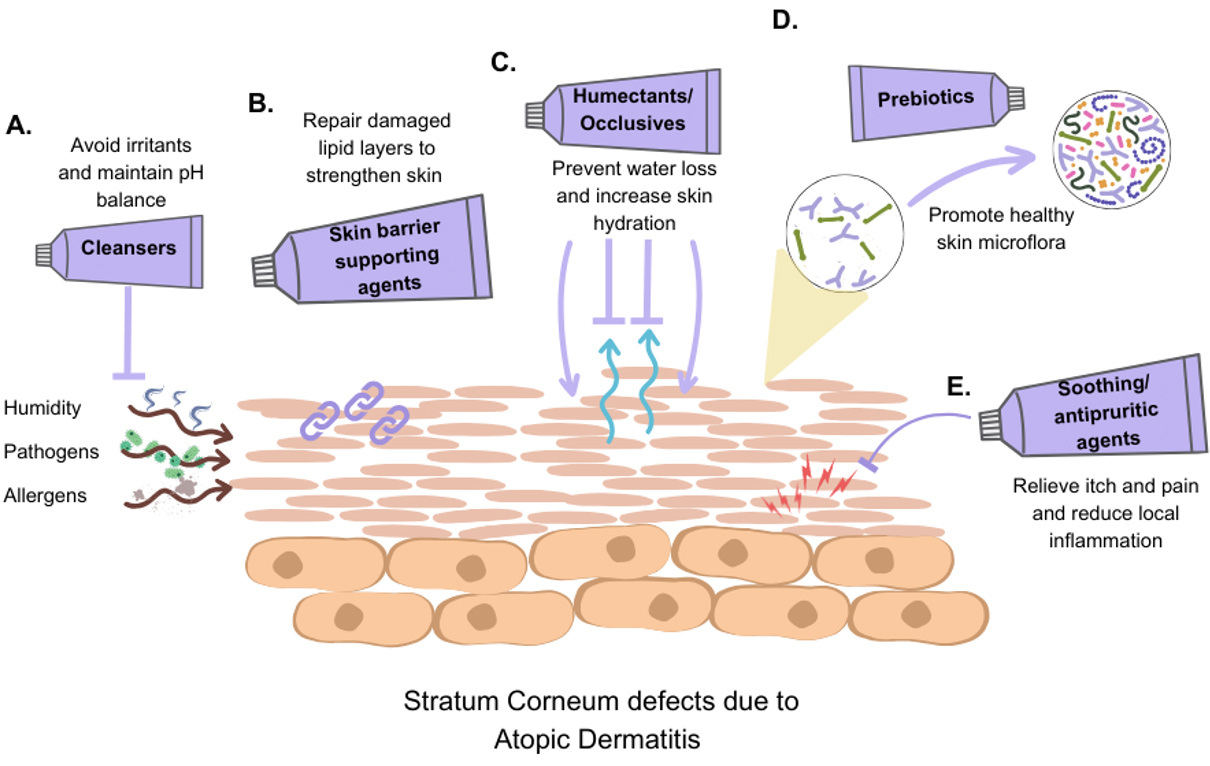
Active ingredients improve skin barrier function in AD. A schematic overview of therapeutic strategies in AD management. It illustrates how active ingredients, such as (A) pH-balancing cleansers, (B) skin barrier supporting agents, (C) humectants and occlusives, (D) prebiotics, and (E) soothing/antipruritic agents, contribute to restoring skin barrier function and improving overall skin health in AD management.
3.2. Promoting Beneficial Skin Microflora Balance Through Topical Prebiotics
It is still unclear whether topical prebiotics have a positive impact on dysbiosis in AD patients, mainly because these ingredients are often included in more complex formulations in studies. The literature shows variability and conflicting findings about their effectiveness [78]. Nevertheless, dermatologists recognise that promoting microbial equilibrium is a key element of AD therapies and favour the development of skincare products for AD that can preserve a balanced and healthy skin microbiome [79]. A recent in vitro study indicated that a 5% concentration of galacto-oligosaccharides has a significant stimulating effect on S. epidermidis while exhibiting inhibitory effects on S. aureus, and these effects were observed in both nutrient broth and cosmetic formulations [80]. In an in vitro study, xylitol, a naturally occurring sugar alcohol, was shown to inhibit the growth of S. aureus and Cutibacterium acnes at a concentration of 5% commonly used in skin products, while promoting the growth of S. epidermidis at a lower concentration (1%) [81]. Even though these findings suggest xylitol can modulate skin microbiota selectively, further research is necessary to explore how this modulation impacts immune system functioning in the skin. Growing evidence suggests that prebiotics like galacto-oligosaccharides and xylitol may aid restoration of a healthy skin microbiome and deter colonisation with pathogenic species in individuals with AD (Fig. 2) [82-84] .
4. SUPPORTING EFFECTIVE SELF-CARE FOR AD MANAGEMENT
In alignment with major dermatology guidelines and position statements, we propose that practical and effective AD self-care encompasses avoiding triggers, proper infection control, adherence to recommended treatment regimens (when required), and a simple daily self-care routine [8, 20-24].
Avoiding environmental triggers is crucial for AD patients due to their compromised skin barrier and underlying inflammation. Triggers vary among individuals and may include certain foods, environmental allergens, pollution, harsh fabrics, extreme temperatures, excessive sweating, and stress [30, 85]. For example, air pollution, such as exposure to particulate matter and volatile organic compounds, can disrupt the skin microbiome and barrier, exacerbating inflammation and dryness [86]. Since complete avoidance of environmental triggers is unrealistic, effective infection control, such as regular cleansing, is critical for preventing pathogen entry in AD patients with a weakened skin barrier [36]. However, selecting appropriate cleansers can be challenging, as certain surfactants may cause irritation or disrupt skin pH [87]. Therefore, it is advisable for AD patients to use “gentle” cleansers containing non-ionic and amphoteric surfactants [21].
Since flares may still occur, it is essential for patients to adhere to their prescribed treatment regimens. Delayed initiation, misapplication, or premature termination of prescribed treatments can aggravate the condition [15, 88]. Evidence also suggests that those with moderate to severe AD and frequent relapses may benefit from proactive therapy, which involves scheduled, intermittent, long-term use of TCS or CNIs on previously affected skin alongside daily moisturisation, though this is beyond the scope of this paper [89].
Adherence, whether to treatment regimens or self-care practices, can be challenging for many patients due to the complexity of routines and concerns about treatment side effects. Simplifying self-care approaches can help patients integrate these practices more easily into daily routines, potentially improving adherence and long-term outcomes [21, 22, 69]. PCPs can play an important role in long-term AD management by providing patients with reliable information and practical advice for day-to-day skin care and protection. We see two important and complementary roles for PCPs in supporting patients with AD. First, while advising patients on appropriate medication use and promoting medication adherence (e.g., to prescribed topical therapies), PCPs can help reinforce the benefits of daily emollient-based skincare routine, as recommended in professional guidelines [8, 20-24]. Second, they can help patients navigate the wide variety of available skincare products by recommending formulations or ingredients for AD management that are supported by scientific evidence [59, 90].
A key aspect of this guidance is educating patients on the correct quantity and frequency of product application. For example, the fingertip unit (FTU) corresponds to approximately 0.5 grams of topical product when squeezed from a standard tube with a 5 mm nozzle from the tip of an adult’s index finger to the first crease [91]. The FTU method provides a simple, consistent guide to ensure proper dosing for effective treatment (e.g., about 2.5 FTUs are required to adequately cover the entire face and neck for adults). Additionally, PCPs can educate patients on tailoring their skincare routines to different AD phases. When no skin lesions are present, patients should focus on gentle cleansing and skin barrier protection as part of the daily care regimen. During increased lesion activity, emollients with soothing agents can be introduced alongside continued gentle cleansing and skin barrier protection. In flare-ups, it is crucial to initiate prescribed treatments while maintaining a regimen that relieves itchiness, gently cleanses, and provides skin barrier protection on the affected areas. As flare-ups resolve, patients can return to their regular gentle cleansing and skin barrier protection routine to support long-term skin health and comfort (Fig. 3).
5. CLEANSE, CONTROL, CALM: IMPLEMENTING A SIMPLE SYNERGISTIC APPROACH TO AD MANAGEMENT
To facilitate effective consultations with AD patients, here we present an easy-to-follow 3C (Cleanse, Control, Calm) approach for daily mild to moderate eczema skin care routine [8, 20-24] (Fig. 4).
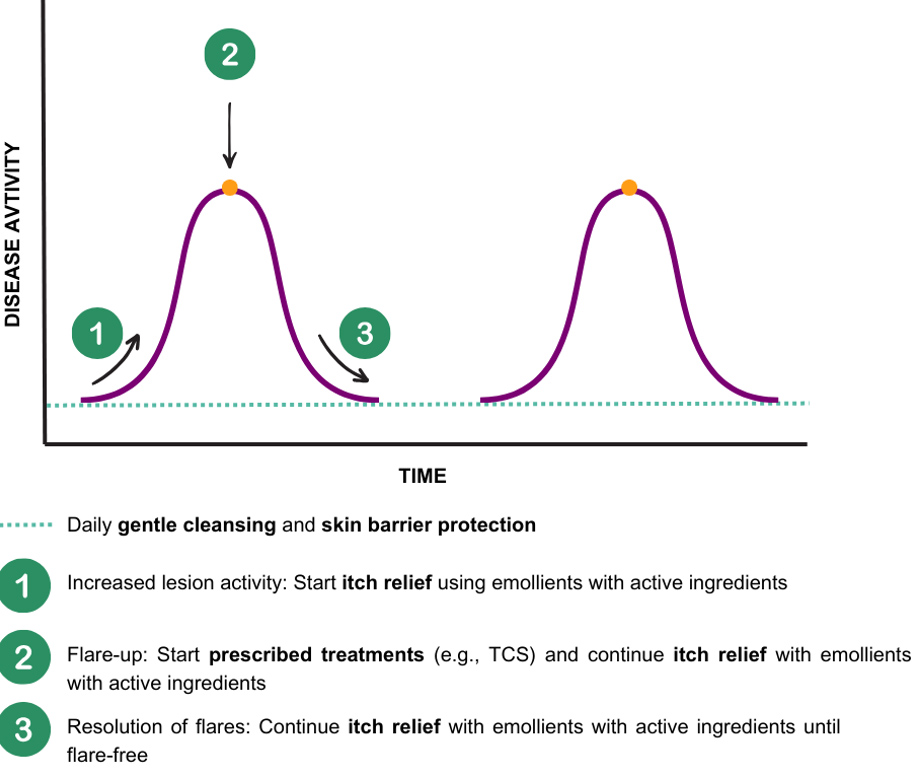
Holistic skin care during and outside of flare-up periods. Holistic skin care is an essential part of AD management throughout and outside of AD flare-ups. Holistic skincare emphasises (1) the need for itch relief using emollients with active ingredients during increased lesion activity, (2) the initiation of prescribed treatments during flare-ups while continuing itch relief, and (3) the continuation of itch relief into the resolution phase until completely flare-free. It highlights the importance of regular gentle cleansing and skin barrier protection throughout all stages to maintain long-term skin health.

3C concept: simple, sustainable self-care for long-term AD management. An overview of the 3C (Cleanse, Control, Calm) approach as a simple daily skincare regimen for AD patients aimed at supporting longer flare-free periods, maintaining skin barrier integrity, and managing the itch-scratch cycle.
5.1. Cleanse
Proper cleansing is a critical step in AD management as it helps remove allergens, irritants, and harmful bacteria from the skin, reducing the risk of flare-ups and infections [8, 20-22, 92]. A prospective study found that using a gentle cleanser containing non-ionic and amphoteric-based surfactants and other supportive active ingredients resulted in significantly greater skin surface hydration after 29 days than water alone (5.16 vs 3.65 arbitrary units; p = 0.011). Notably, the gentle cleanser did not affect the skin’s pH, microbiome biodiversity, or TEWL compared with the control group treated with water only [93]. The advantages of gentle cleansing for AD were demonstrated in a randomised controlled trial, in which the use of body wash formulations containing lipids and zinc pyrithione for four weeks was associated with significant reductions in S. aureus colonisation, improved microbial diversity, reduced corticosteroid usage, and clinically meaningful improvements in SCORAD scores compared with baseline [94]. These data suggest that gentle cleansers can help maintain healthy skin hydration and contribute to improved skin health and symptom control.
Harsh soaps or cleansers containing fragrances and other potential irritants should be avoided by AD patients as they can disrupt the skin barrier and potentially worsen AD symptoms [87, 92]. Moreover, according to Thai guidelines, it is advisable to maintain the water temperature between 27–30°C to prevent skin dehydration during bathing. Additionally, it is recommended to limit bathing time to 5–10 minutes, followed by a gentle drying of the skin [92]. PCPs should guide patients in choosing gentle, pH-balanced cleansers that do not strip the skin of its natural lipids, thus minimising skin irritation and dryness. Patients may also be encouraged to select cleansers with emollient or moisturising ingredients to provide added hydration and help soothe dry, itchy skin [21].
5.2. Control
Achieving control of AD requires preserving the integrity of the skin barrier, promoting healthy skin microflora, and preventing the entry of irritants and allergens, which can trigger or promote inflammation. The core of this approach is the consistent use of emollients to maintain skin hydration and a healthy lipid lamellar structure, thereby enhancing overall skin function [69, 95]. Two randomised studies evaluated the effect of an emollient formulation containing active ingredients to strengthen the skin barrier and support healthy skin microflora. The use of the emollient formulation containing these ingredients reduced TEWL significantly (N = 23, -168.36 vs. -123.38 g/m2/h, p = 0.023), improved skin hydration, and increased mean intercellular lipid lamellae length from baseline (day 22: 120.61 vs. 35.85 nm/1000nm2, p < 0.001). Additionally, the formulation did not adversely affect bacterial viability [96]. Furthermore, a separate study evaluated the effectiveness and safety of a topical emollient containing panthenol in a 3-month maintenance treatment regimen for infants and children. This research revealed that the treatment group exhibited a significant reduction in AD severity scores, achieving a notably higher success rate (i.e., the proportion of subjects without flares at the study end) compared with the reference group (99% vs 77%, p = 0.083), suggesting that regular use of skin care products may contribute to the prevention or reduction of AD flare-ups [97]. Therefore, regular use of emollients with active ingredients in an AD management routine can help maintain skin health and promote long-term control of symptoms.
Hence, patients with mild to moderate AD may be recommended to use emollients on a daily basis, together with gentle cleansing [8, 20-24]. Furthermore, in addition to recommending suitable products, PCPs should be mindful that the recommended emollient dosage varies based on individual factors, such as age and extent of skin involvement. For example, dermatology guidelines recommend emollient application of up to 200 g/week for young children and up to 500 g/week for adults [20, 92]. Therefore, PCPs should offer guidance to patients following these age-specific recommendations. As a practical point, the FTU method can help patients accurately gauge the amount of emollient needed for effective eczema management. One FTU (~ 0.5 g) of emollient is sufficient for application to two adult palm areas, corresponding to approximately 2% of an adult’s body surface area [20].
5.3. Calm
Using products specifically formulated to aid in soothing and healing AD lesions is important during flare-ups but also beneficial within a daily skincare regimen [8, 20-24]. In a blinded, randomised pilot trial, an emollient preparation containing soothing agents, including dexpanthenol and glycyrrhetinic acid, demonstrated significant improvements in TEWL, skin hydration, and itch relief compared with no treatment [98]. In another double-blinded, randomised trial, an emollient containing soothing agents, including licochalcone A, significantly reduced relapse rates within 12 weeks compared with the vehicle arm (28.6% vs 71.4%; p < 0.01). The results demonstrated the emollient’s ability to maintain barrier homeostasis while effectively reducing itch [99]. These studies support using itch-relieving ingredients alongside an emollient-based skincare regimen as needed during flare-ups to promote recovery.
Accordingly, when AD patients experience flares, PCPs may advise patients to use topical treatment and emollients with soothing active ingredients (Table 1). However, if the symptoms persist, patients should seek advice from a dermatologist. To effectively manage flare-ups, it is important to remind patients to refrain from scratching or picking at affected areas and promptly treat any signs of infection [8, 20-24].
CONCLUSION
Emollients play a crucial role in the long-term management of AD. Recent evidence strongly supports the inclusion of specific active ingredients in emollient formulations for daily skin care, which have been shown to reduce the frequency of flare-ups with regular use. Ingredients such as physiological lipids, prebiotics, soothing agents, and humectants address key pathophysiological processes in AD and have demonstrated efficacy in improving skin barrier integrity, enhancing hydration, relieving itch, and restoring the health of the skin microbiome. Using emollient products containing these ingredients can provide much-needed relief and help AD patients break the itch-scratch cycle. Combining such skin care regimens with other proactive self-care measures, such as avoiding environmental triggers and practising effective infection control, may help patients achieve better long-term control of their condition.
Here, we proposed a simple, evidence-based eczema care routine that can serve as a resource for PCPs managing patients with mild to moderate AD. This 3C approach incorporates ingredients known to be effective in restoring and maintaining a healthy skin barrier in AD patients. Emollients form the foundation of daily skincare and can be used alongside topical therapies to enhance effectiveness. However, in cases where flare-ups persist and prove difficult to control, patients should be referred to a dermatologist for specialised care. Cleanse highlights the significance of gently removing environmental irritants and allergens while preserving the skin’s natural lipids and physiological pH levels. Control emphasises the daily use of key ingredients that strengthen and maintain a healthy skin barrier. Calm addresses the management of flare-ups as needed by applying formulations that contain soothing ingredients to alleviate symptoms and support skin healing. Following this daily routine and utilising the identified beneficial ingredients can help patients break the itch-scratch cycle and manage their condition more effectively in the long term.
The 3C framework is derived from principles and evidence-based practices recommended in various guidelines and supported by studies; however, we acknowledge that it has not been directly evaluated as a structured regimen for self-care in patients with mild to moderate AD. Further research is needed to assess how it may influence adherence and outcomes in real-world practice.
Daily self-care forms the foundation of holistic approaches to AD management, supporting the use of active treatment regimens as required for individual patients. From the healthcare professionals’ perspective, staying up to date with emerging therapy options and other complementary aspects of AD management is essential. One of these aspects is comprehensive patient support, which may include regular follow-ups to improve adherence to both prescribed treatments and self-care routines, addressing the long-term psychological impacts of AD, and structured education programs that are patient-centred and culturally sensitive. Although a detailed discussion of these aspects is beyond the scope of this paper, we recognise the need for a holistic approach that integrates medical, psychological, and social support to improve the quality of care for individuals living with AD.
AUTHORS’ CONTRIBUTIONS
S.W.: W.L.: W.W.: R.N.: S.C: participated in conceptualisation, writing, reviewing, and editing of the manuscript.
LIST OF ABBREVIATIONS
| AD | = Atopic Dermatitis |
| PCP | = Primary Care Practitioners |
| TCS | = Topical Corticosteroids |
| CNI | = Calcineurin Inhibitors |
| JAK | = Janus Kinase |
| UVB | = Ultraviolet B |
| SCORAD | = SCORing Atopic Dermatitis |
| TEWL | = Transepidermal Water Loss |
| SC | = Stratum Corneum |
| FTU | = Fingertip Unit |
ACKNOWLEDGEMENTS
Medical writing and editorial support were provided by Tech Observer Asia Pacific Pte. Ltd.


